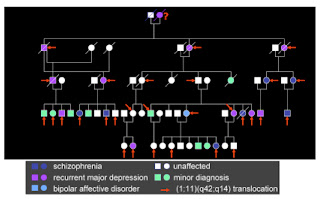
What is a gene "for"?

“Scientists discover gene for autism” (or ovarian cancer, or depression, cocaine addiction, obesity, happiness, height, schizophrenia… and whatever you’re having yourself). These are typical newspaper headlines (all from the last year) and all use the popular shorthand of “a gene for” something. In my view, this phrase is both lazy and deeply misleading and has caused widespread confusion about what genes are and do and about their influences on human traits and disease. The problem with this phrase stems from the ambiguity in what we mean by a “gene” and what we mean by “for”. These can mean different things at different levels and unfortunately these meanings are easily conflated. First, a gene can be defined in several different ways. From a molecular perspective, it is a segment of DNA that codes for a protein, along with the instructions for when and where and in what amounts this protein should be made. (Some genes encode RNA molecules, rather than proteins, but the g