Split brains, autism and schizophrenia
A new study suggests that a gene known to be causally linked to schizophrenia and other psychiatric disorders is involved in the formation of connections between the two hemispheres of the brain. DISC1 is probably the most famous gene in psychiatric genetics, and rightly so. It was discovered in a large Scottish pedigree, where 18 members were affected by psychiatric disease.
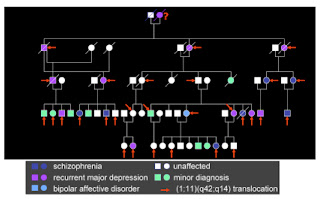 The diagnoses ranged from schizophrenia and bipolar disorder to depression and a range of “minor” psychiatric conditions. It was found that the affected individuals had all inherited a genetic anomaly – a translocation of genetic material between two chromosomes. This basically involves sections of two chromosomes swapping with each other. In the process, each chromosome is broken, before being spliced back to part of the other chromosome. In this case, the breakpoint on chromosome 1 interrupted a gene, subsequently named Disrupted-in-Schizophrenia-1, or DISC1.
The diagnoses ranged from schizophrenia and bipolar disorder to depression and a range of “minor” psychiatric conditions. It was found that the affected individuals had all inherited a genetic anomaly – a translocation of genetic material between two chromosomes. This basically involves sections of two chromosomes swapping with each other. In the process, each chromosome is broken, before being spliced back to part of the other chromosome. In this case, the breakpoint on chromosome 1 interrupted a gene, subsequently named Disrupted-in-Schizophrenia-1, or DISC1.
That this discovery was made using classical “cytogenetic” techniques (physically looking at the chromosomes down a microscope) and in a single family is somehow pleasing in an age where massive molecular population-based studies are in vogue. (A win for “small” science).
The discovery of the DISC1 translocation clearly showed that disruption of a single gene could lead to psychiatric disorders like schizophrenia. This was a challenge to the idea that these disorders were “polygenic” – caused by the inheritance in each individual of a large number of genetic variants. As more and more mutations in other genes are being found to cause these disorders, the DISC1 situation can no longer be dismissed as an exception – it is the norm.
It also was the first example of a principle that has since been observed for many other genes – namely that the effects of the mutation can manifest quite variably - not as one specific disorder, but as different ones in different people. Indeed, DISC1 has since been implicated in autism as well as adult-onset disorders. It is now clear from this and other evidence that these apparently distinct conditions are best thought of as variable outcomes that arise, in many cases at least, from disturbances of neurodevelopment.
Since the initial discovery, major research efforts of a growing number of labs have been focused on the next obvious questions: what does DISC1 do? And what happens when it is mutated? What happens in the brain that can explain why psychiatric symptoms result?
We now know that DISC1 has many different functions. It is a cytoplasmic protein - localised inside the cell - that interacts with a very large number of other proteins and takes part in diverse cellular functions, including cell migration, outgrowth of nerve fibres, the formation of dendritic spines (sites of synaptic contact between neurons), neuronal proliferation and regulation of biochemical pathways involved in synaptic plasticity. Many of the proteins that DISC1 interacts with have also been implicated in psychiatric disease.
This new study adds another possible function, and a dramatic and unexpected one at that. This function was discovered from an independent angle, by researchers studying how the two hemispheres of the brain get connected – or more specifically, why they sometimes fail to be connected. The cerebral hemispheres are normally connected by millions of axons which cross the midline of the brain in a structure called the corpus callosum (or “tough body” – (don’t ask)). Very infrequently, people are born without this structure – the callosal axons fail to cross the midline and the two hemispheres are left without this major route of communication (though there are other routes, such as the anterior commissure).
The frequency of agenesis of the corpus callosum has been estimated at between 1 in 1,000 and 1 in 6,000 live births – thankfully very rare. It is associated with a highly variable spectrum of other symptoms, including developmental delay, autistic symptoms, cognitive disabilities extending into the range of mental retardation, seizures and other neurological signs.
 Elliott Sherr and colleagues were studying patients with this condition, which is very obvious on magnetic resonance imaging scans (see Figure). They initially found a mother and two children with callosal agenesis who all carried a deletion on chromosome 1, at position 1q42 – exactly where DISC1 is located. They subsequently identified another patient with a similar deletion, which allowed them to narrow down the region and identify DISC1 as a plausible candidate (among some other genes in the deleted region). Because the functions of proteins can be affected not just by large deletions or translocations but also by less obvious mutations that change a single base of DNA, they also sequenced the DISC1 gene in a cohort of callosal agenesis patients and found a number carrying novel mutations that are very likely to disrupt the function of the gene.
Elliott Sherr and colleagues were studying patients with this condition, which is very obvious on magnetic resonance imaging scans (see Figure). They initially found a mother and two children with callosal agenesis who all carried a deletion on chromosome 1, at position 1q42 – exactly where DISC1 is located. They subsequently identified another patient with a similar deletion, which allowed them to narrow down the region and identify DISC1 as a plausible candidate (among some other genes in the deleted region). Because the functions of proteins can be affected not just by large deletions or translocations but also by less obvious mutations that change a single base of DNA, they also sequenced the DISC1 gene in a cohort of callosal agenesis patients and found a number carrying novel mutations that are very likely to disrupt the function of the gene.
While not rock-solid evidence that it is DISC1 that is responsible, these data certainly point to it as the strongest candidate to explain the callosal defect. This hypothesis is strongly supported by findings from DISC1 mutant mice (carrying a mutation that mimics the effect of the human translocation), which also show defects in formation of the corpus callosum. In addition, the protein is strongly expressed in the axons that make up this structure at the time of its development.
The most obvious test of whether disruption of DISC1 really causes callosal agenesis is to look in the people carrying the initial translocation. Remarkably, it is not known whether the original patients in the Scottish pedigree who carry the DISC1 translocation show this same obvious brain structural phenotype. They have, very surprisingly, never been scanned.
This new paper raises the obvious hypothesis that the failure to connect the two hemispheres results in the psychiatric or cognitive symptoms, which variously include reduced intellectual ability, autism and schizophrenia. This seems like too simplistic an interpretation, however. All we have now is a correlation. First, the implication of DISC1 in the acallosal phenotype is not yet definitive – this must be nailed down and replicated. But even if it is shown that disruption of DISC1 causes both callosal agenesis and schizophrenia (or other psychiatric disorders or symptoms), this does not prove a causal link. DISC1 has many other functions and is expressed in many different brain areas (ubiquitously in fact). Any, or indeed, all of these functions may in fact be the cause of psychopathology.
One prediction, if it were true that the lack of connections between the two hemispheres is causal, is that we would expect the majority of patients with callosal agenesis to have these kinds of psychiatric symptoms. In fact, the rates are indeed very high – in different studies it has been estimated that up to 40% of callosal agenesis patients have an autism diagnosis, while about 8% have the symptoms of schizophrenia or bipolar disorder. (Of course, these patients may have other, less obvious brain defects as well, so even this is not definitive).
Conversely, we might naively expect a high rate of callosal agenesis in patients with autism or schizophrenia. However, we know these disorders are extremely heterogeneous and so it is much more likely that this phenotype might be apparent in only a specific (possibly very small) subset of patients. This may indeed be the case – callosal agenesis has been observed in about 3 out of 200 schizophrenia patients (a vastly higher rate than in the general population). Another study, just published, has found that mutations in a different gene – ARID1B – are also associated with callosal agenesis, mental retardation and autism. More generally, there may be subtle reductions in callosal connectivity in many schizophrenia or autism patients (including some autistic savants).
Whether this defect can explain particular symptoms is not yet clear. For the moment, the new study provides yet another possible function of DISC1, and highlights an anatomical phenotype that is apparently present in a subset of autism and schizophrenia cases and that can arise due to mutation in many different genes (of which DISC1 and ARID1B are only two of many known examples).
One final note: formation of the corpus callosum is a dramatic example of a process that is susceptible to developmental variation. What I mean is this: when patients inherit a mutation that results in callosal agenesis, this phenotype occurs in some patients but not all. This is true even in genetically identical people, like monozygotic twins or triplets (or in lines of genetically identical mice). Though the corpus callosum contains millions of nerve fibres, the initial events that establish it involve very small numbers of cells. These cells, which are located at the medial edge of each cerebral hemisphere, must contact each other to enable the fusion of the two hemispheres, forming a tiny bridge through which the first callosal fibres can cross. Once these are across, the rest seem able to follow easily. Because this event involves very few cells at a specific time in development, it is susceptible to random “noise” – fluctuations in the precise amounts of various proteins in the cells, for example. These are not caused by external forces – the noise is inherent in the system. The result is that, in some people carrying such a mutation the corpus callosum will not form at all, while in others it forms apparently completely normally (see figure of triplets, one on left with normal corpus callosum, the other two with it absent). So, an all-or-none effect can arise, without any external factors involved.
The result is that, in some people carrying such a mutation the corpus callosum will not form at all, while in others it forms apparently completely normally (see figure of triplets, one on left with normal corpus callosum, the other two with it absent). So, an all-or-none effect can arise, without any external factors involved.
This same kind of intrinsic developmental variation may also explain or at least contribute to the variability in phenotypic outcome at the level of psychiatric symptoms when these kinds of neurodevelopmental mutations are inherited. Even monozygotic twins are often discordant for psychiatric diagnoses (concordance for schizophrenia is about 50%, for example). This is often assumed to be due to non-genetic and therefore “environmental” or experiential factors. If these disorders really arise from differences in brain wiring, which we know are susceptible to developmental variation, then differences in the eventual phenotype could actually be completely intrinsic and innate.
Osbun N, Li J, O'Driscoll MC, Strominger Z, Wakahiro M, Rider E, Bukshpun P, Boland E, Spurrell CH, Schackwitz W, Pennacchio LA, Dobyns WB, Black GC, & Sherr EH (2011). Genetic and functional analyses identify DISC1 as a novel callosal agenesis candidate gene. American journal of medical genetics. Part A, 155 (8), 1865-76 PMID: 21739582
Halgren C, Kjaergaard S, Bak M, Hansen C, El-Schich Z, Anderson CM, Henriksen KF, Hjalgrim H, Kirchhoff M, Bijlsma EK, Nielsen M, den Hollander NS, Ruivenkamp CA, Isidor B, Le Caignec C, Zannolli R, Mucciolo M, Renieri A, Mari F, Anderlid BM, Andrieux J, Dieux A, Tommerup N, & Bache I (2011). Corpus Callosum Abnormalities, Mental Retardation, Speech Impairment, and Autism in Patients with Haploinsufficiency of ARID1B. Clinical genetics PMID: 21801163
 The diagnoses ranged from schizophrenia and bipolar disorder to depression and a range of “minor” psychiatric conditions. It was found that the affected individuals had all inherited a genetic anomaly – a translocation of genetic material between two chromosomes. This basically involves sections of two chromosomes swapping with each other. In the process, each chromosome is broken, before being spliced back to part of the other chromosome. In this case, the breakpoint on chromosome 1 interrupted a gene, subsequently named Disrupted-in-Schizophrenia-1, or DISC1.
The diagnoses ranged from schizophrenia and bipolar disorder to depression and a range of “minor” psychiatric conditions. It was found that the affected individuals had all inherited a genetic anomaly – a translocation of genetic material between two chromosomes. This basically involves sections of two chromosomes swapping with each other. In the process, each chromosome is broken, before being spliced back to part of the other chromosome. In this case, the breakpoint on chromosome 1 interrupted a gene, subsequently named Disrupted-in-Schizophrenia-1, or DISC1.
That this discovery was made using classical “cytogenetic” techniques (physically looking at the chromosomes down a microscope) and in a single family is somehow pleasing in an age where massive molecular population-based studies are in vogue. (A win for “small” science).
The discovery of the DISC1 translocation clearly showed that disruption of a single gene could lead to psychiatric disorders like schizophrenia. This was a challenge to the idea that these disorders were “polygenic” – caused by the inheritance in each individual of a large number of genetic variants. As more and more mutations in other genes are being found to cause these disorders, the DISC1 situation can no longer be dismissed as an exception – it is the norm.
It also was the first example of a principle that has since been observed for many other genes – namely that the effects of the mutation can manifest quite variably - not as one specific disorder, but as different ones in different people. Indeed, DISC1 has since been implicated in autism as well as adult-onset disorders. It is now clear from this and other evidence that these apparently distinct conditions are best thought of as variable outcomes that arise, in many cases at least, from disturbances of neurodevelopment.
Since the initial discovery, major research efforts of a growing number of labs have been focused on the next obvious questions: what does DISC1 do? And what happens when it is mutated? What happens in the brain that can explain why psychiatric symptoms result?
We now know that DISC1 has many different functions. It is a cytoplasmic protein - localised inside the cell - that interacts with a very large number of other proteins and takes part in diverse cellular functions, including cell migration, outgrowth of nerve fibres, the formation of dendritic spines (sites of synaptic contact between neurons), neuronal proliferation and regulation of biochemical pathways involved in synaptic plasticity. Many of the proteins that DISC1 interacts with have also been implicated in psychiatric disease.
This new study adds another possible function, and a dramatic and unexpected one at that. This function was discovered from an independent angle, by researchers studying how the two hemispheres of the brain get connected – or more specifically, why they sometimes fail to be connected. The cerebral hemispheres are normally connected by millions of axons which cross the midline of the brain in a structure called the corpus callosum (or “tough body” – (don’t ask)). Very infrequently, people are born without this structure – the callosal axons fail to cross the midline and the two hemispheres are left without this major route of communication (though there are other routes, such as the anterior commissure).
The frequency of agenesis of the corpus callosum has been estimated at between 1 in 1,000 and 1 in 6,000 live births – thankfully very rare. It is associated with a highly variable spectrum of other symptoms, including developmental delay, autistic symptoms, cognitive disabilities extending into the range of mental retardation, seizures and other neurological signs.
 Elliott Sherr and colleagues were studying patients with this condition, which is very obvious on magnetic resonance imaging scans (see Figure). They initially found a mother and two children with callosal agenesis who all carried a deletion on chromosome 1, at position 1q42 – exactly where DISC1 is located. They subsequently identified another patient with a similar deletion, which allowed them to narrow down the region and identify DISC1 as a plausible candidate (among some other genes in the deleted region). Because the functions of proteins can be affected not just by large deletions or translocations but also by less obvious mutations that change a single base of DNA, they also sequenced the DISC1 gene in a cohort of callosal agenesis patients and found a number carrying novel mutations that are very likely to disrupt the function of the gene.
Elliott Sherr and colleagues were studying patients with this condition, which is very obvious on magnetic resonance imaging scans (see Figure). They initially found a mother and two children with callosal agenesis who all carried a deletion on chromosome 1, at position 1q42 – exactly where DISC1 is located. They subsequently identified another patient with a similar deletion, which allowed them to narrow down the region and identify DISC1 as a plausible candidate (among some other genes in the deleted region). Because the functions of proteins can be affected not just by large deletions or translocations but also by less obvious mutations that change a single base of DNA, they also sequenced the DISC1 gene in a cohort of callosal agenesis patients and found a number carrying novel mutations that are very likely to disrupt the function of the gene.
While not rock-solid evidence that it is DISC1 that is responsible, these data certainly point to it as the strongest candidate to explain the callosal defect. This hypothesis is strongly supported by findings from DISC1 mutant mice (carrying a mutation that mimics the effect of the human translocation), which also show defects in formation of the corpus callosum. In addition, the protein is strongly expressed in the axons that make up this structure at the time of its development.
The most obvious test of whether disruption of DISC1 really causes callosal agenesis is to look in the people carrying the initial translocation. Remarkably, it is not known whether the original patients in the Scottish pedigree who carry the DISC1 translocation show this same obvious brain structural phenotype. They have, very surprisingly, never been scanned.
This new paper raises the obvious hypothesis that the failure to connect the two hemispheres results in the psychiatric or cognitive symptoms, which variously include reduced intellectual ability, autism and schizophrenia. This seems like too simplistic an interpretation, however. All we have now is a correlation. First, the implication of DISC1 in the acallosal phenotype is not yet definitive – this must be nailed down and replicated. But even if it is shown that disruption of DISC1 causes both callosal agenesis and schizophrenia (or other psychiatric disorders or symptoms), this does not prove a causal link. DISC1 has many other functions and is expressed in many different brain areas (ubiquitously in fact). Any, or indeed, all of these functions may in fact be the cause of psychopathology.
One prediction, if it were true that the lack of connections between the two hemispheres is causal, is that we would expect the majority of patients with callosal agenesis to have these kinds of psychiatric symptoms. In fact, the rates are indeed very high – in different studies it has been estimated that up to 40% of callosal agenesis patients have an autism diagnosis, while about 8% have the symptoms of schizophrenia or bipolar disorder. (Of course, these patients may have other, less obvious brain defects as well, so even this is not definitive).
Conversely, we might naively expect a high rate of callosal agenesis in patients with autism or schizophrenia. However, we know these disorders are extremely heterogeneous and so it is much more likely that this phenotype might be apparent in only a specific (possibly very small) subset of patients. This may indeed be the case – callosal agenesis has been observed in about 3 out of 200 schizophrenia patients (a vastly higher rate than in the general population). Another study, just published, has found that mutations in a different gene – ARID1B – are also associated with callosal agenesis, mental retardation and autism. More generally, there may be subtle reductions in callosal connectivity in many schizophrenia or autism patients (including some autistic savants).
Whether this defect can explain particular symptoms is not yet clear. For the moment, the new study provides yet another possible function of DISC1, and highlights an anatomical phenotype that is apparently present in a subset of autism and schizophrenia cases and that can arise due to mutation in many different genes (of which DISC1 and ARID1B are only two of many known examples).
One final note: formation of the corpus callosum is a dramatic example of a process that is susceptible to developmental variation. What I mean is this: when patients inherit a mutation that results in callosal agenesis, this phenotype occurs in some patients but not all. This is true even in genetically identical people, like monozygotic twins or triplets (or in lines of genetically identical mice). Though the corpus callosum contains millions of nerve fibres, the initial events that establish it involve very small numbers of cells. These cells, which are located at the medial edge of each cerebral hemisphere, must contact each other to enable the fusion of the two hemispheres, forming a tiny bridge through which the first callosal fibres can cross. Once these are across, the rest seem able to follow easily. Because this event involves very few cells at a specific time in development, it is susceptible to random “noise” – fluctuations in the precise amounts of various proteins in the cells, for example. These are not caused by external forces – the noise is inherent in the system.
 The result is that, in some people carrying such a mutation the corpus callosum will not form at all, while in others it forms apparently completely normally (see figure of triplets, one on left with normal corpus callosum, the other two with it absent). So, an all-or-none effect can arise, without any external factors involved.
The result is that, in some people carrying such a mutation the corpus callosum will not form at all, while in others it forms apparently completely normally (see figure of triplets, one on left with normal corpus callosum, the other two with it absent). So, an all-or-none effect can arise, without any external factors involved.
This same kind of intrinsic developmental variation may also explain or at least contribute to the variability in phenotypic outcome at the level of psychiatric symptoms when these kinds of neurodevelopmental mutations are inherited. Even monozygotic twins are often discordant for psychiatric diagnoses (concordance for schizophrenia is about 50%, for example). This is often assumed to be due to non-genetic and therefore “environmental” or experiential factors. If these disorders really arise from differences in brain wiring, which we know are susceptible to developmental variation, then differences in the eventual phenotype could actually be completely intrinsic and innate.
Osbun N, Li J, O'Driscoll MC, Strominger Z, Wakahiro M, Rider E, Bukshpun P, Boland E, Spurrell CH, Schackwitz W, Pennacchio LA, Dobyns WB, Black GC, & Sherr EH (2011). Genetic and functional analyses identify DISC1 as a novel callosal agenesis candidate gene. American journal of medical genetics. Part A, 155 (8), 1865-76 PMID: 21739582
Halgren C, Kjaergaard S, Bak M, Hansen C, El-Schich Z, Anderson CM, Henriksen KF, Hjalgrim H, Kirchhoff M, Bijlsma EK, Nielsen M, den Hollander NS, Ruivenkamp CA, Isidor B, Le Caignec C, Zannolli R, Mucciolo M, Renieri A, Mari F, Anderlid BM, Andrieux J, Dieux A, Tommerup N, & Bache I (2011). Corpus Callosum Abnormalities, Mental Retardation, Speech Impairment, and Autism in Patients with Haploinsufficiency of ARID1B. Clinical genetics PMID: 21801163



Thanks a lot for this review!
ReplyDeleteIt is surprising though that the absence of the corpus callosum, which is a super-large structure in our brains is only causing very mild phenotypes, at least in mice. Even in humans, some people seems to be doing fine without it.
The Corpus Callosum being a recent innovation, present only in eutherian mammals, I was wondering if the phenotypical disparities you are describing could be explained by the fact that this structure didn't had the time to evolve a truly original and vital function, and is still redondant with the other major commissures of the forebrain?
An interesting, and related, point:
ReplyDeleteIt appears that the corpus callosum appeared in evolving mammals at the same time (or perhaps shortly after) as a major miRNA cluster; at least both appear to be present in all eutherian ("placental") mammals and absent in all other vertebrates:
"In this study, we describe a detailed analysis of the miRNA cluster (hereafter mir-379/mir-656 cluster) located within the imprinted DLK-DIO3 region on human chromosome 14. We show that orthologous miRNA clusters are present in all sequenced genomes of the placental (eutherian) mammals but not in the marsupial (metatherian), monotreme (prototherian), or any other vertebrate genomes. We provide evidence that the locus encompassing this cluster emerged in an early eutherian ancestor prior to the radiation of modern placental mammals by tandem duplication of the ancient precursor sequence. The original amplified cluster may have contained in excess of 250 miRNA precursor sequences, most of which now appear to be inactive. Examination of the eutherian genomes showed that the cluster has been maintained in evolution for approximately 100 Myr.
Analysis of genes that contain predicted evolutionarily conserved targets for miRNAs from this cluster revealed significant overrepresentation of the Gene Ontology terms associated with biological processes such as neurogenesis, embryonic development, transcriptional regulation, and RNA metabolism. Consistent with these findings, a survey of the miRNA expression data within the cluster demonstrates a strong bias toward brain and placenta samples from adult organisms and some embryonic tissues.
Our results suggest thatemergence of the mir-379/mir-656 miRNAclusterwas one of the factors that facilitated evolution of the placental mammals. Overrepresentation of genes involved in regulation of neurogenesis among predicted miRNAs targets indicates an important role of the mir-379/mir-656 cluster in this biological process in the placental mammals."
Glazov, E.A., McWilliam, S., Barris, W.C., Dalrymple, B.P. (2008) Origin, Evolution, and Biological Role of miRNA Cluster in DLK-DIO3 Genomic Region in Placental Mammals Mol. Biol. Evol. 25(5):939–948. 2008 doi:10.1093/molbev/msn045
Just wanted to know how do antipsychotics bridge this gap and help patients with psychosis?
ReplyDeleteI really like to know about the forms and ways that the brain uses to work, I am in a experiment in xl pharmacy about the unknown power of the human brain !
ReplyDeleteI have noticed that if there is one in a family there is usually the other...
ReplyDeleteWe are having a discussion on this
www.mforums.org
In many articles listed with PubMed & The National Institute of Health, it's been proven that the parasite Toxoplasma Gondii are the cause of Bi-Polar & Schizophrenia. Go here for a complete article:
ReplyDeletehttp://efherne.wordpress.com/2012/05/27/100/
There is definitely so many people that are suffering with these disorders. So much more research has to be done with these so much. This is causing so many issues with do many people. So dangerous to have. Skunk Removal Toronto
ReplyDeleteAerophobia, or fear of flying, may be associated with numerous other phobias. Sometimes it appears on its own. The fear of flying is estimated to affect as many as 25% of people, although a full- blown phobia is significantly less common. Travel delays, common when flying at popular times, can make the fear of flying worse. Whether or not your fear of flying has developed into a phobia, it can have devastating effects on your quality of life.
ReplyDeleteLern more about psychiatry.... psychiatry , Aerophobia
As a mathematician, I find this subject fascinating. The lack (or diminished) of connectivity between the cerebral hemisphere is what graph theorists call a "graph cut". In network theory, making a "graph cut" alters the behavior of the network radically in a beneficial or a detrimental way as the case may be. Hopefully my question is not too naive but being an outsider to this field, I wonder if many people have studied from a mathematical graph theory perspective the effect of "graph cuts" (in the sense I just described) on the human brain.
ReplyDeleteReferences welcome!