Popping the hood on synaesthesia – what’s going on in there?
-->Synaesthesia – a “mixing of the senses” –
was a popular scientific topic in the late 19th century, but fell
out of favour during the mid-20th century, mainly due to the
influence of behaviorism, which held that subjective experience was not a
suitable subject for serious science. The start of this century has seen
resurgence in interest in the topic, partly fuelled by the hope that neuroimaging
would provide objective measures of what is happening in the brains of people
during synaesthetic experiences.
We (Erik O’Hanlon, Fiona Newell and myself)
have recently published our own neuroimaging study of synaesthesia, combining
structural and functional analyses. Some of what follows is pulled from that
paper, which contains references to the many studies cited below. Many of the
ideas below are also discussed in a chapter I wrote for the new Oxford Handbook of Synaesthesia: Synaesthesia
and cortical connectivity – a neurodevelopmental perspective.
The term synaesthesia refers both to the
experience of some kind of cross-activation from one sense to another (but see
more below) and to the condition of being prone to such experiences. It can be
caused acutely in some people by drugs like LSD or psilocybin, which can
famously induce visual experiences in response to music. It can also arise, very rarely, due to brain injuries, which leave one part of the brain without its normal
innervation, causing invasion of neighbouring nerve fibres and a rewiring of
the source of activation of a region, while the “meaning” of its activation
remains the same.
Both of those types differ in many aspects
from what is known as developmental synaesthesia. This is a heritable condition
in which particular stimuli generate specific and consistent additional sensory
percepts or associations in another sensory modality or processing stream. Easy
for me to say, I know – that is such a mouthful because it has to encompass
many different forms. These include seeing letters or words in colour or
associating them with colours, seeing colours in response to sounds (typically words
or music), tasting words, feeling tastes as tactile sensation, associating
numbers or calendar units with spatial locations and many others. It is surprisingly
common, with between 1 and 4% of the population estimated to have the condition.
Though originally defined as a cross-sensory phenomenon, many cases involve more
conceptual inducing stimuli (“inducers”) or resultant percepts (“concurrents”).
Synaesthesia may thus be better thought of as the association of additional
attributes into what some psychologists call the “schema” of the inducing
object. Thus, the schema of the letter “A” would incorporate not only its
particular shapes and sounds, but also the fact that it is, say, olive-green.
Middle C may smell of oranges, Wednesday may be located behind a person’s head
and the word “shed” may taste of boiled cabbage – these kinds of associations
are idiosyncratic but highly stable in individual synaesthetes.
The mechanism driving these additional
percepts or associations is unknown, though most researchers agree it is likely
related to functions in the cerebral cortex. This is the part of the brain where
specialised areas emerge that are dedicated to processing the kinds of stimuli
that often induce synaesthetic experiences or associations – such as letters,
words, musical notes, numbers, calendar units. These are specialised
categories, each with many different members, which are learned through experience.
As a child has repeated exposures to stimuli such as letters, a particular part
of the visual cortex becomes specialised for processing them, showing more and
more selective responses for letters with greater experience. That region not
only becomes more responsive to letters, it becomes less responsive to other
stimuli. Also, learning has to sharpen the representations of each letter, so
that all the various forms of the letter “A” are recognised as such, while
simultaneously being distinguished from “D”, “R”, and other visually similar
graphemes. In addition, the shapes for “A” have to be linked to the various
sounds that it can make, in different contexts.
In contrast to the inducing stimuli, the
concurrent percepts associated with the synaesthetic experience tend to be much
simpler: colours, tastes, textures, spatial locations. These perceptual
primitives are also typically processed by specialised circuits or areas of the
cortex, but ones that mature much earlier and that develop in a manner that is
not so strictly driven by experience. For example, a particular area of the
visual cortex, called V4, is selectively involved in processing colour: this
area is strongly activated by coloured stimuli; if it is stimulated with an
electrode, patches of colour may be seen in the visual field; and, finally,
damage to V4 can lead to complete colour blindness.
So, a simple model for what is happening in
synaesthesia is that activation of one cortical area by an inducing stimulus
(say, letters), aberrantly and consistently causes co-activation of another
cortical area (say, the colour area), leading to an additional colour percept
or association. Neuroimaging seems like the perfect way to test this hypothesis
– if true, we should be able to see additional areas “lighting up” in
functional magnetic resonance imaging (fMRI) scans when synaesthetes are
exposed to stimuli that induce a synaesthetic experience.
By now, about a dozen functional neuroimaging experiments have been performed to try to define the neural
correlates of synaesthetic experiences. Most of these have studied subjects
with grapheme-colour or sound-colour synaesthesia and many have looked
specifically for activation of V4 or other visual areas in response to the
presentation of the “inducer” – either aurally presented sounds or visually
presented achromatic graphemes. These have indeed provided some insights into
the neural basis of synaesthesia but their findings are surprisingly variable.
Some of them have reported exactly the
expected observation – extra activation of regions such as V4. However, it is
not at all clear that such an effect can be taken as a ground truth, as other
studies have not observed this but have seen activation or functional
connectivity differences in other visual areas or in other brain regions, such
as parietal cortex. Still others have observed no additional activation
correlating with the synaesthetic experience at all. One early positron emission tomography (PET) study even found, in addition to some areas of extra
activation in coloured-hearing synaesthetes, greater cortical deactivation in other areas in response
to spoken words that induced a synaesthetic experience of colour.
What is going on in the brain of
synaesthetes during a synaesthetic experience thus remains very much an open
question. Phenotypic heterogeneity may explain some of the variation in these
results – perhaps all of the results are “right” and mechanisms differ across
synaesthetes in different studies. Even if that is the case, a simple model of
excess cross-activation between highly restricted cortical areas seems too
minimal to accommodate all these findings. Rather, these findings suggest that
differences in connectivity may be quite extensive in the brains of
synaesthetes, a hypothesis which is supported by structural neuroimaging
studies.
These studies have been performed to try
and identify anatomical correlates of the condition
of synaesthesia (as opposed to the fMRI experiments which are looking at the experience of synaesthesia). They aimed
to test the hypothesis that cortical modularity breaks down in people with
synaesthesia due to the presence of additional anatomical connections between
normally segregated cortical areas. (The alternative type of model proposes altered
neurochemistry, leading to disinhibition of normally existing connections).
Here, the findings are somewhat more
consistent, at least on a general level. Several studies have now identified
structural differences in the brains of synaesthetes compared to controls. In
almost all cases, synaesthetes showed greater volumes of areas of grey or white
matter or greater "fractional anisotropy" within certain white matter tracts than
controls. Some of these differences are in the general region of visual areas
thought to be involved in the synaesthetic experience but others are more
widespread, in parietal or even frontal regions. A recent study analysed global
connectivity patterns in the brains of synaesthetes, using networks derived
from correlations in cortical thickness. The global network topology was
significantly different between synaesthetes and controls, with synaesthetes
showing increased clustering, suggesting global hyperconnectivity. The
differences driving these effects were widespread and not confined to areas
hypothesised to be involved in the grapheme-colour synaesthetic experience
itself. Widespread functional connectivity differences have also been observed
in a study using resting-state fMRI.
There is thus a strong general trend: the
brains of groups of synaesthetes do show structural differences to those of
groups of controls, these are concentrated in occipital and temporal regions
but extend also to parietal and frontal lobes, and they almost always involve
increases in the measured parameters in synaesthetes. Though the exact
locations of such differences vary between studies, the fact that they all
agree in the direction of the effects strongly argues that they represent a
real, generalizable finding.
If only we knew what it meant. It could
mean that the primary cause of synaesthesia is really a structural difference
in the brain. However, the imaging parameters measured (like volume of some cluster
of grey matter or fractional anisotropy of a white matter tract) are really
quite crude and influenced by many variables at a cellular neuroanatomical
level. What has not yet emerged is tractography evidence showing an example of
connections that are clearly not present in non-synaesthetes. It is thus not
obvious how the observed structural differences can explain the synaesthetic
experience. It could just as well be that structural differences
are secondary and arise due to a lifetime of altered activity patterns in the
neural circuits involved. Or the structural differences might be entirely
unrelated to the experience of synaesthesia and reflect instead some broader
phenotypes associated with the condition.
With this as background, we designed a
neuroimaging study aimed at probing the functional involvement in the
synaesthetic experience of areas with structural differences. What we found
surprised us.
We compared a group of 13 synaesthetes with
a group of 11 controls (decent sample sizes for this field, but more on that
below). First we looked for average structural differences between the members
of these two groups. Using a method called voxel-based morphometry, we
identified multiple clusters of increased volume of either grey or white matter
in the synaesthetes compared to controls. We also used diffusion-weighted imaging to look at the structural parameters of nerve fibres and found multiple
regions of increased fractional anisotropy in synaesthetes compared to
controls. Similar to previous studies, these structural differences were
concentrated in but not exclusive to the back of the brain (occipital and
temporal lobes) and were all increases in synaesthetes.
So far, so good – these results generally
replicate and extend previous findings. We then used fMRI to investigate how
the areas showing a structural difference responded to stimuli that induce a
synaesthetic experience. All the synaesthetes in the study had grapheme-colour
synaesthesia – they attribute colours to letters of the alphabet. We showed
them images of letters or of non-meaningful characters, as a contrast, and
examined responses in nine areas of increased grey matter volume. Four of those
areas showed a differential response to this contrast, in synaesthetes but not
in controls (a “group by condition interaction”).
When we looked more closely at the
responses in these areas we found something really surprising. Two of them
showed a clear difference in response to letters, but this was driven by a very
strong reduction in activity in
synaesthetes. Not only was the BOLD (blood oxygen level-dependent) signal lower
than in controls, it was lower than baseline in those voxels. There is good
evidence that negative BOLD signals of this type reflect cortical deactivations – a suppression of
neuronal activity in that region. None of the areas showed a greater response
to letters in synaesthetes.
We also performed an unbiased, whole-brain
analysis with the same contrast, again expecting to find regions with an
increased selective response to letters in synaesthetes. We found fourteen
areas showing a group by condition interaction, but none of these were driven
by increased activation to letters in synaesthetes. Three of them were driven
by negative BOLD responses in synaesthetes (these did not overlap the areas
with grey matter volume differences).
What does this all mean?
My first thought, and I hope it is yours
too, is: “possibly nothing”. After all, these are unexpected results from
exploratory analyses. While they are corrected for multiple tests, they still
could represent a false positive observation – a statistical blip that occurred
in that experiment, with that sample, that does not represent a generalizable
finding. This is a problem that dogs the fMRI literature and there is only one
solution to it – replication, replication, replication! Because our study was
designed as at least a conceptual replication and extension of previous
findings, we did not include a separate replication sample. (It was honestly
also partly because the field does not demand it). If we were designing a
similar study today, I would certainly aim for a larger sample and an
independent replication sample (and would hope that funding agencies would
begin to apply these standards more rigorously).
Actually though, this finding is not
completely novel – cortical deactivations were previously reported in response
to synaesthesia-inducing stimuli in a PET study, some in the same areas we
observe. Whether they have occurred in other fMRI studies is a little hard to
know – experimental designs focusing on specific regions or looking
specifically for positive differences may have missed these kinds of effects.
The idea that cortical deactivations might
be involved in synaesthetic experiences is also neither unprecedented nor
outlandish. Here’s what we say in the paper:
“One possible, though
speculative, explanation for these observations relates to the fact that the
synaesthetic percept or association is internally generated and often reported
as being “in the mind’s eye”. A number of studies have shown that generation of
an internal sensory representation induces deactivation of regions which might
compete for attention or provide conflicting information. For example, visual
imagery induces negative BOLD in auditory cortex, verbal memory induces
deactivation across auditory and visual cortices and imagery of visual motion
induces deactivation of early visual cortices (V1-3). Amedi and colleagues
found a strong correlation across subjects between the deactivation of auditory
cortex during visual mental imagery and their score on the vividness of visual
imagery questionnaire (VVIQ). We have previously reported that synaesthetes
tend to score higher on this imagery measure. This is not to suggest that the
synaesthetic percepts arise from the same processes as mental imagery per se –
there is evidence from functional imaging that this is not the case. But it is
possible that the vividness of a mental image and of a synaesthetic percept
both rely on deactivation of other areas.
Such a conclusion is supported by findings
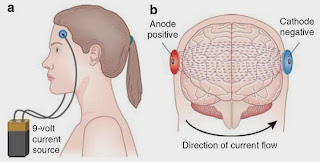
from a transcranial direct current stimulation (tDCS) study.
[This technique basically hooks up a 9-volt
battery to electrodes on your scalp, and applies small zaps of current in
particular patterns. It can be applied to affect particular regions and to
either activate them or inhibit them. Activating motor cortex can cause muscle
movements while activating visual cortex can cause perception of winking lights
or “phosphenes” in the visual field].
Terhune and colleagues found that
synaesthetes showed enhanced cortical excitability of primary visual cortex,
with a 3-fold lower phosphene detection threshold in response to activation by
tCDS. [This finding is consistent with a previous study from our own group
using electroencephalography, which found that the amplitude of early visual
evoked potentials was larger in synaesthetes compared to controls, even in
response to very simple visual stimuli that did not induce a synaesthetic
experience].
They tested whether this hyperexcitability
of primary cortex could be either a contributing source to the generation of
the synaesthetic percept, or, alternatively, a competing signal, which would
interfere with the conscious perception of the synaesthetic percept. They show
strong evidence that the latter is the case – stimulation or inhibition of
primary visual cortical activity diminished or enhanced, respectively, the
synaesthetic experience, based on both self-reports and behavioural
interference measures. It thus seems plausible that the cortical deactivations
we observe in response to stimuli that induce the synaesthetic experience could
be an important part of that response, possibly involved in reducing the
signals of competing percepts and allowing the internally generated
synaesthetic percept to reach conscious awareness.”
Future studies will hopefully tell whether
these kinds of cortical deactivations really are an important component of the
synaesthetic experience. For now, our findings add to a quite varied set of
neuroimaging findings, which have yet to definitively nail down the neural
correlates of the synaesthetic experience. Perhaps expecting a single mechanism
is a mistake – if the condition is really heterogeneous we may need some other
means (like genetics perhaps) to segregate subjects and elucidate the neural
underpinnings of this fascinating condition.






Fascinating stuff! I would love to be considered for a future study, and would love this broken down into even more layperson's terms. :)
ReplyDeleteCould we activate visual cortex well enough to send images to the patient?
ReplyDeleteThis comment has been removed by a blog administrator.
ReplyDeleteHave I got this correct? You and colleagues were surprised to find evidence of cortical deactivations in the synaesthetes?
ReplyDeleteWhen I was told about synesthesia, I was shocked to find out that not everyone was like me when it came to stuff like time, and numbers-- not everyone saw these as having shapes and colors. That realization caused me to become fully aware of when time(months, years, days, etc.)/numbers "manifest" before me, whereas before I was so accustomed to it because I thought it normal. Furthermore, I also realized then, that there I also see hours and minutes, and that is when I found it really annoying and distracting. But, I've grown used to it again, I know it just happens, so I accept it, instead of trying to ignore or fight it. I look up the various forms of synesthesia, especially mine (sequence-space-synesthesia, or conceptual synesthesia, or time-space synesthesia) to help myself understand why our brains are doing this. I hope that extra research in synesthesia can lead to stronger understanding of the human brain in general.
ReplyDelete